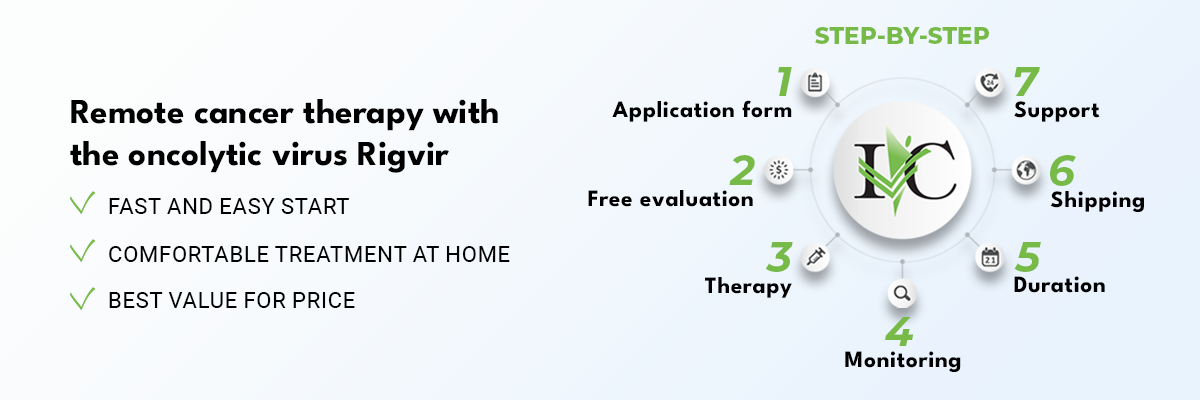
Oncolytic virus preclinical toxicology studies
Read
more

Stage IIA Skin Melanoma Treatment With ECHO-7 Oncolytic Virus Rigvir
Read more

4-Week repeated dose rat GLP toxicity study of oncolytic ECHO-7 virus
Rigvir administered intramuscularly with a 4-week recovery period
Read more

Clinical landscape of oncolytic virus research in 2020
Read more

The Oncolytic Virus in Cancer Diagnosis and Treatment
Read more

A progressive stage IIIB melanoma treated with oncolytic ECHO-7 virus: A
case report
Read more

Management of a primary malignant melanoma of uterine cervix stage IVA
patient with radical surgery and adjuvant oncolytic virus Rigvir® therapy: A case report
Read more

Adrenal Gland and Gastric Malignant Melanoma without Evidence of Skin
Lesion Treated with the Oncolytic Virus Rigvir
Read more

Effect of oncolytic ECHO-7 virus strain Rigvir on uveal melanoma cell
lines
Read more

ECHO-7 oncolytic virus Rigvir® in an adjuvant setting for stage
I uveal melanoma; A retrospective case report
Read more

Treatment of a stage III rima glottidis patient with the oncolytic virus
Rigvir.
Read
more

Combination treatment with nivolumab and Rigvir of a progressive stage IIC
skin melanoma patient.
Read more

A Case of Stage IV Chromophobe Renal Cell Carcinoma Treated with the
Oncolytic ECHO-7 Virus, Rigvir®
Read more

Oncolytic viruses are a fast-developing cancer treatment field. Numerous
viruses have been tested in clinical trials and three are approved. The first, Rigvir, is an
immunomodulator with anti-tumour effect
Read more

Multimodality Treatment of a Colorectal Cancer Stage IV Patient with
FOLFOX-4, Bevacizumab, Rigvir Oncolytic Virus, and Surgery
Read more

Effect of the oncolytic ECHO-7 virus Rigvir® on the viability
of cell lines of human origin in vitro
Read more

Melanoma Unknown Primary Brain Metastasis Treatment with ECHO-7 Oncolytic
Virus Rigvir: A Case Report
Read
more

Long-term treatment with the oncolytic ECHO-7 virus Rigvir of a melanoma
stage IV M1c patient, a small cell lung cancer stage IIIA patient, and a histiocytic sarcoma stage
IV patient-three case reports
Read more

Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs
survival in melanoma patients after surgical excision of the tumor in a retrospective study
Read more
![]()